The course consists of four independent, interactive modules that are designed to help the biomedical research community—including researchers, NIH grant applicants, and peer reviewers—account for and appropriately integrate sex as a biological variable (SABV) across the full spectrum of biomedical sciences. The NIH SABV policy originated in a notice titled “Consideration of Sex as a Biological Variable in NIH-funded Research.” It states NIH’s expectation that SABV will be factored into research designs, analyses, and reporting in vertebrate animal and human studies as scientifically appropriate.
Dr. Janine Austin Clayton, SABV Introduction
Dr. Chyren Hunter, SABV Primer Overview
As biomedical scientists and health professionals, we know that good science is important, and we know that those working in a field that can affect the health of an individual aim to provide the best care possible. That's why the consideration of SABV is a key focus of the NIH initiative to enhance reproducibility in biomedical research through rigor and transparency in studies. The better the science, the better able we might be able to identify the critical evidence that can improve health for everyone.
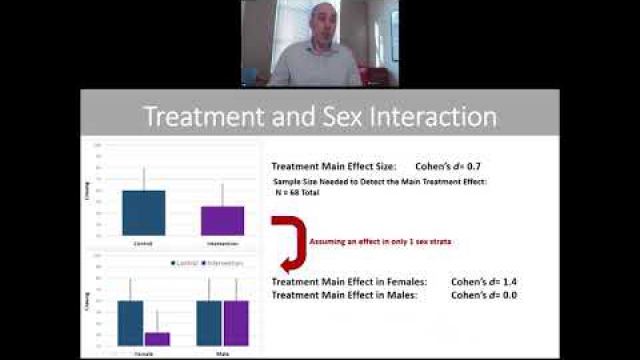
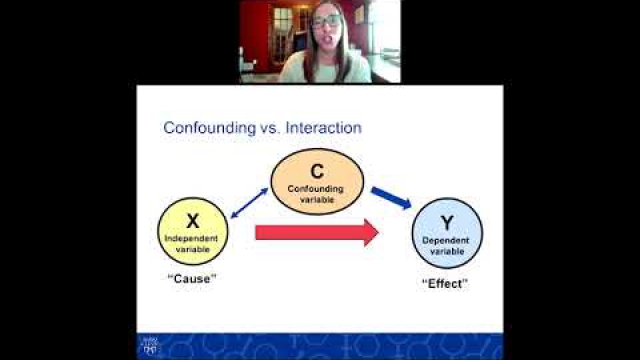
Considering SABV in the experimental design of biomedical research is essential to ensuring rigor and transparency and to improving generalizability of findings.
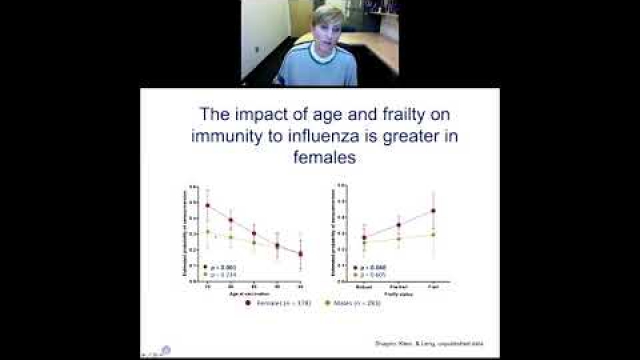
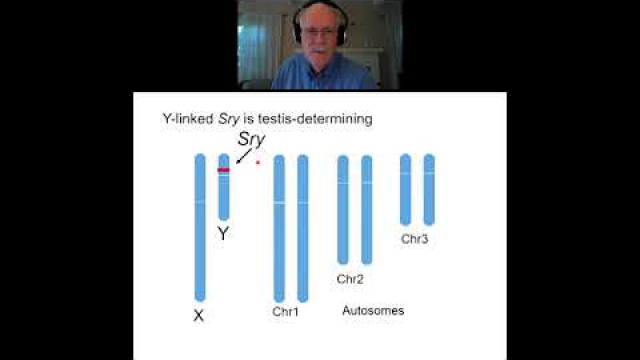
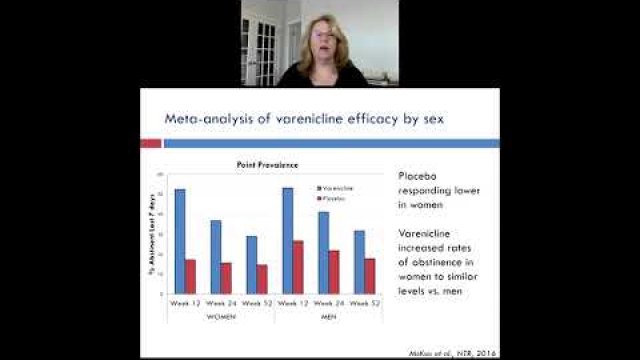
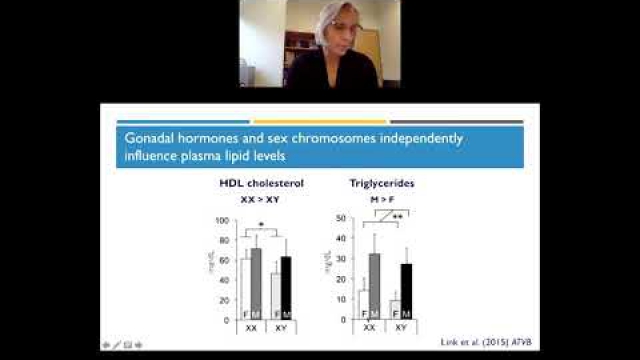
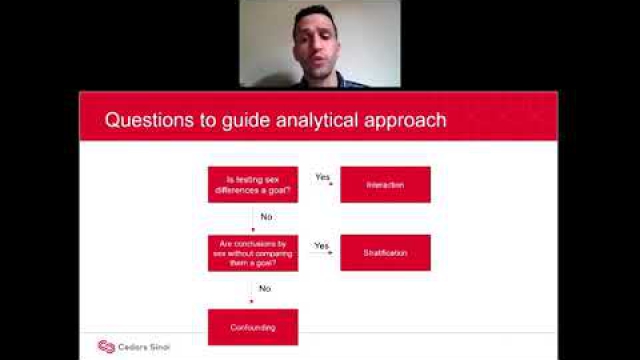
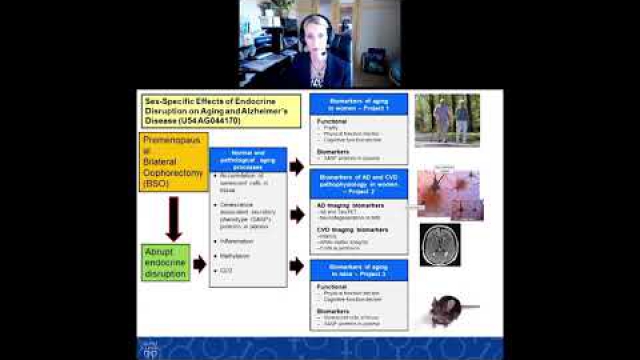
A key component of the consideration of SABV includes characterizing and analyzing sex-based data.
Full transparency in reporting experimental details, data, and results, including sex-specific reporting, ensures that the biomedical community knows to whom the results of your research apply.
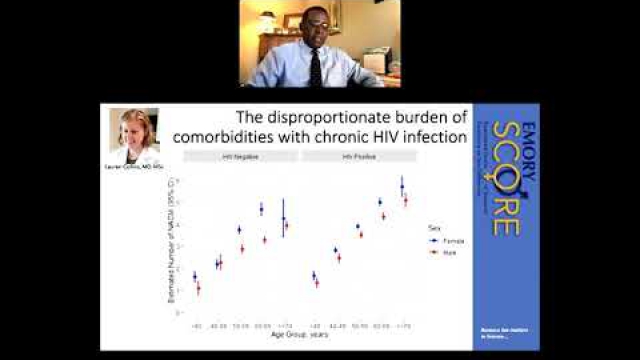
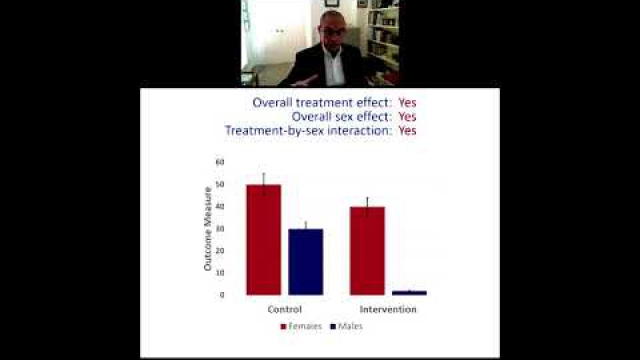
In preclinical and clinical work that aims to develop therapeutic interventions, sex-specific reporting is also essential to determining whether benefits or adverse events differ by sex.