NIH Administrative Supplement Programs Support
Early-Career Biomedical Investigators
The NIH Working Group on Women in Biomedical Careers developed two notices of special interest (NOSIs) of administrative supplements to support and enhance the retention of early-career biomedical investigators during critical life events.
The first NOSI, Administrative Supplements to Promote Research Continuity and Retention of NIH Mentored Career Development (K) Award Recipients and Scholars (currently NOT-OD-23-031), announced a program to support junior investigators who have received K awards as they transition from individual mentored career development to research independence. This program aims to improve retention and minimize departures from the biomedical research workforce. The second NOSI, Administrative Supplement for Continuity of Biomedical and Behavioral Research Among First-Time Recipients of NIH Research Project Grant Awards (currently NOT-OD-23-032), announced a program designed to enhance the retention of investigators who are transitioning to the first renewal of their first independent Research Project Grant award or to a second NIH Research Project Grant award. Retention at the first renewal or continuous NIH Research Project Grant support is crucial for both sustaining the ongoing research NIH has made an investment in the biomedical research workforce.
Eligibility for these supplement programs hinges on the Program Director/Principal Investigator (PD/PI) experiencing a critical life event such as childbirth, adoption, having serious personal health issues (e.g., an illness and/or a debilitating condition), having a high-risk pregnancy, or having primary caregiving responsibilities for an ailing spouse, child, partner, parent or a member of one’s immediate family during the project. Supplements provide up to $70,000 in direct costs for the PD/PI to maintain the progress of research while the PD/PI is affected by the critical life event. The most common uses of the funds include hiring additional personnel, such as research assistants and coordinators, and buying supplies and equipment. The rate of funding for requested supplements is 65%.
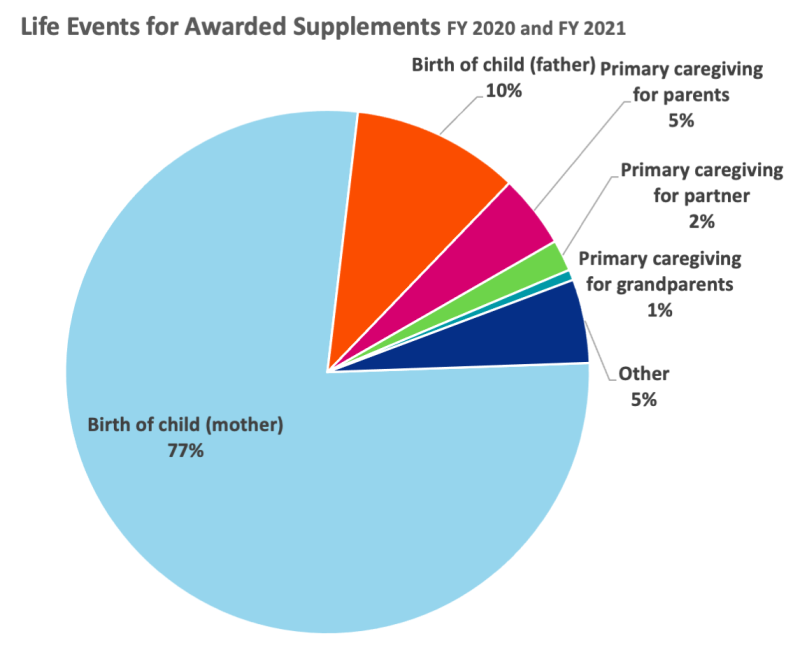
Figure 1. Cited reasons for applicants requesting funding to support the continuation of their research or their retention in the biomedical workforce. Since the inception of the programs in FY 2020, childbirth has been the number one reason for the administrative supplements.
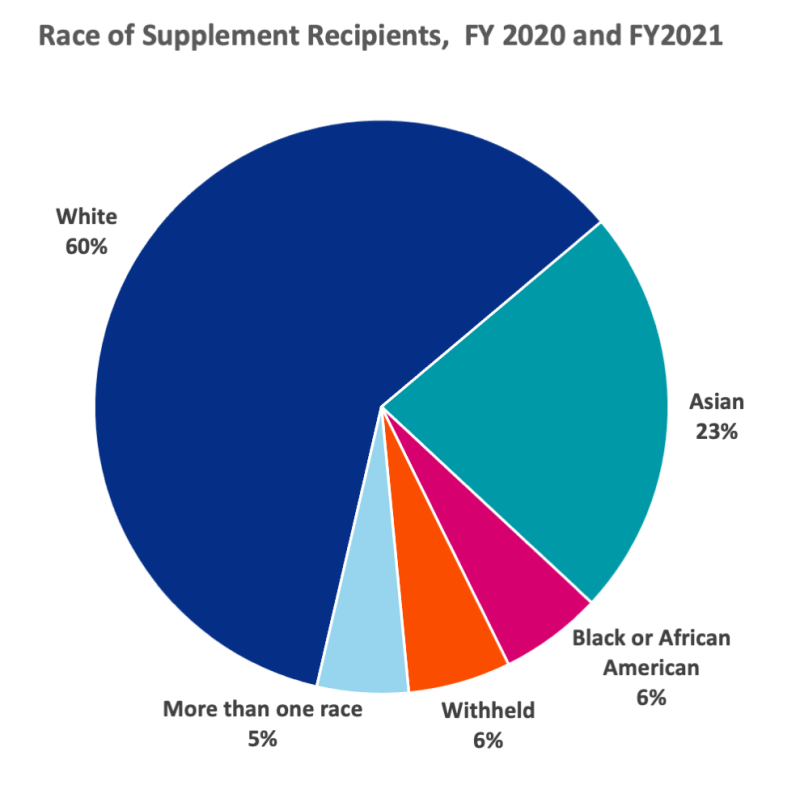
Figure 2. Distribution of the race of supplement recipients. Since the inception of the programs in 2020, most of the recipients of the two continuity and retention supplements have been White (60%).
Potential applicants can find details at the following links, and NIH Institute and Center-specific information can be found in the respective NOSIs.
- NOT-OD-23-031
- NOT-OD-23-032
- NIH Grants & Funding
- FAQs about NIH’s family-friendly initiatives

