OADR-ORWH Funding Information
The autoimmune disease research portfolio encompasses more than 140 different conditions categorized across 15 disease areas, many of which intersect multiple NIH Institute and Center (IC) mission areas. Central to OADR-ORWH’s mission is the facilitation of collaborative autoimmune disease research across ICs, which includes support for extramural and intramural high-priority research. OADR-ORWH supports new and existing research awards focused on multi-IC initiatives, interdisciplinary research teams, and innovative science to accelerate the diagnosis and treatment of autoimmune diseases.
Looking for NIH Funding Opportunities relevant to autoimmune diseases?
OADR-ORWH FY23-24 Co-Funding
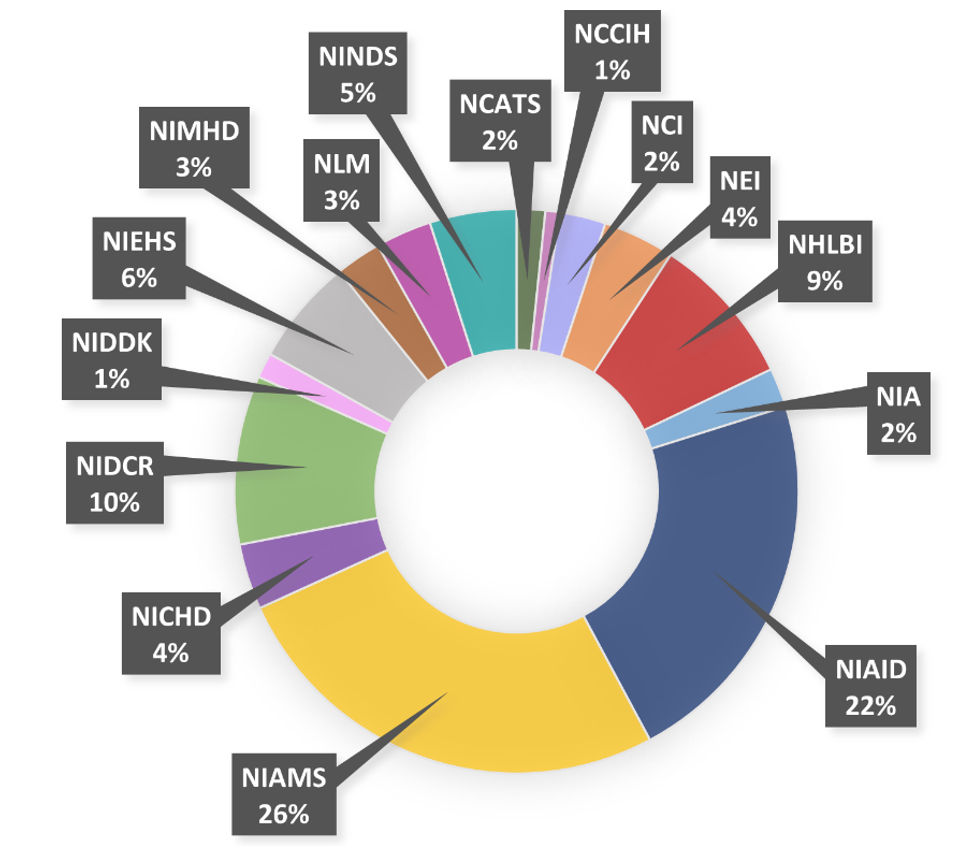
Figure A. Combined distribution of OADR-ORWH
co-funds across 15 NIH ICs for FY23 and FY24.
Click image to enlarge.
In Fiscal Year (FY) 2023 and FY24, OADR-ORWH co-funded 92 awards that included 54 extramural research awards, 7 R56 bridge awards, 18 intramural research awards, 11 intramural scientific fellowships, 1 workshop, and 1 contract/cooperative agreement. OADR-ORWH strategically supported research across 14 autoimmune disease areas that touched 52 different autoimmune diseases and included immunome, exposome, and data integration research. These awards were administered through 15 different ICs (Figure A) and fulfilled OADR-ORWH’s objectives to support autoimmune disease research across NIH and enhance resources for new and ongoing research.


In FY23 and FY24, OADR-ORWH supported two multi-year awards from the collaborative Accelerating Medicines Partnership® Autoimmune and Immune-Mediated Diseases (AMP® AIM) program that is managed through the Foundation for the NIH along with support from NIAMS, NIDCR, NIAID, NEI, private partners, and not-for-profit organizations. The AMP® AIM program is a partnership that brings together the resources of the public, private, and not-for-profit sectors to pioneer a transformational approach to studying autoimmune diseases at a cellular level, with a focus on identifying new targets for drug development. In FY24, OADR-ORWH also co-funded an additional AMP® AIM award to support the Technology and Analytic Cores (TACs) and Research Management Unit (RMU).

In FY24, OADR-ORWH forged a new collaboration with NIAID’s Autoimmunity Centers of Excellence (ACE) program. This partnership expanded the ACE network, which now includes a new center that is focused on the study of endocrine-specific autoimmunity and five new pilot projects. The ACE program is designed to facilitate collaborative research and accelerate the development of innovative therapies for autoimmune diseases.

In FY24, OADR-ORWH supported the establishment of a new NEXUS: Network for Exposomics in the U.S Coordinating Center in collaboration with NIEHS, NIAMS, NCI, NIA, and NINDS. This coordinating center aims to support the integration of exposomics across a global network and promote interdisciplinary partnerships that seek to better understand how environmental factors impact health outcomes, including autoimmune diseases.
Learn more about FY24 OADR-ORWH Funding:
- R56 bridge awards
- Intramural research awards
- Intramural scientific fellowships
- Mucosal Immunity Workshop
Interested in applying to OADR-ORWH Funding Opportunities? Learn more about applying.
For more information on the NIH Institutes and Center, visit Institutes at NIH.