 Mesh hysteropexy, a surgical treatment for uterovaginal prolapse, results in 3-year postoperative outcomes similar to those of hysterectomy. This finding was reported recently in the Journal of the American Medical Association by Charles W. Nager, M.D., and colleagues of the Study of Uterine Prolapse Procedures - Randomized Trial (SUPeR), a study supported by NIH’s Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) and ORWH.
Mesh hysteropexy, a surgical treatment for uterovaginal prolapse, results in 3-year postoperative outcomes similar to those of hysterectomy. This finding was reported recently in the Journal of the American Medical Association by Charles W. Nager, M.D., and colleagues of the Study of Uterine Prolapse Procedures - Randomized Trial (SUPeR), a study supported by NIH’s Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) and ORWH.
Uterovaginal prolapse is a pelvic floor disorder caused by a weakening of supportive muscles, sometimes resulting in the uterus protruding from the vaginal opening. The condition is most common in older women.
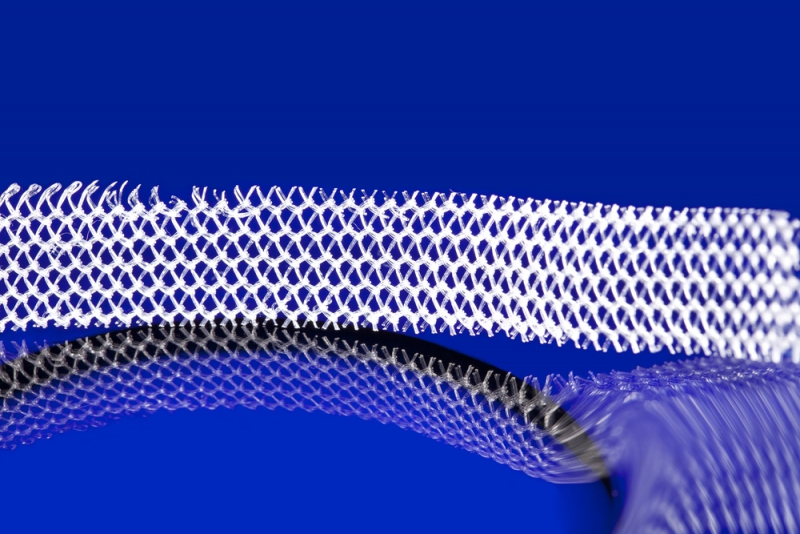
Historically, surgeons have treated uterovaginal prolapse by removing the uterus (called a hysterectomy) and attaching the top of the vagina to deep pelvic ligaments. Mesh hysteropexy represents an alternative treatment, does not require a hysterectomy, and uses a mesh implant similar to the one shown in the photograph to support the uterus.
SUPeR’s primary analysis included 175 patients, 169 of whom (97%) participated in a follow-up 3 years after surgery. Half of the patients were randomized to undergo hysteropexy, and half were randomized to undergo hysterectomy. After 3 years, the two procedures had statistically comparable “failure rates,” that is, a similar number of participants from each treatment group experienced new symptoms of prolapse and/or required retreatment.
Due to some outstanding safety concerns, a U.S. Food & Drug Administration (FDA) safety recall stopped the sale of the mesh kits for uterovaginal prolapse repair in April of 2019, after the surgeries under study had been completed.
SUPeR provides the longest-term comparison to date of the mesh implant and hysterectomy for treating uterovaginal prolapse. Further research is required to determine which procedure is more effective and whether mesh hysteropexy is deemed safe. A follow-up article reporting the outcomes of SUPeR participants at 5 years after surgery is planned.
Reference
Nager et al. 2019. JAMA. 322: 1054-1065.
